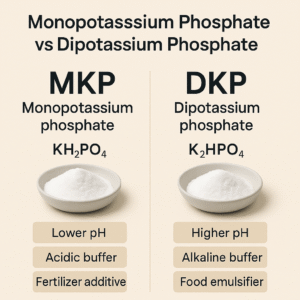
 Monopotassium phosphate (MKP, KH₂PO₄) and dipotassium phosphate (DKP, K₂HPO₄) look very similar on paper: both are potassium phosphates, both supply K and P, and both are widely used in food, beverage, agriculture and industrial formulations.
Monopotassium phosphate (MKP, KH₂PO₄) and dipotassium phosphate (DKP, K₂HPO₄) look very similar on paper: both are potassium phosphates, both supply K and P, and both are widely used in food, beverage, agriculture and industrial formulations.
However, the two salts behave very differently in solution. One is acidic, the other is alkaline. That single difference in acid–base character leads to completely different functions in real applications – from protein stabilization in dairy to leaf spraying in agriculture and pH control in beverages.
This article takes a structured look at MKP vs DKP so that purchasing, R&D, and QA teams can choose the right phosphate for each application and avoid expensive formulation mistakes.
1) Core Chemical Differences Between MKP and DKP
Both MKP and DKP are potassium salts of phosphoric acid, but they correspond to different degrees of neutralization:
| Property | Monopotassium Phosphate (MKP) | Dipotassium Phosphate (DKP) |
|---|---|---|
| Chemical name | Monopotassium phosphate | Dipotassium phosphate |
| Chemical formula | KH₂PO₄ | K₂HPO₄ |
| Neutralization level | Monobasic potassium salt of phosphoric acid | Dibasic potassium salt of phosphoric acid |
| pH of 1% solution (typical) | Acidic, approx. 4.2–4.7 | Weakly alkaline, approx. 8.5–9.6 |
| Acid–base character | Acid phosphate | Basic phosphate |
| Main roles | Acidic buffer, nutrient source, fertilizer P–K source | Alkaline buffer, protein stabilizer, chelator, taste modifier |
In simple terms:
- MKP is an acidic salt – it tends to lower or stabilize pH in the mildly acidic range.
- DKP is an alkaline salt – it tends to raise pH or buffer in the weakly alkaline range.
This difference in pH behavior is the primary reason why the two salts are not interchangeable in formulations, especially where proteins, clarity, taste or microbial stability are critical.
2) Functional Behavior in Solution: Buffering, pH and Proteins
Beyond the basic pH values, MKP and DKP support very different functional roles in aqueous systems.
2.1 Buffering Range and pH Control
The phosphate system has multiple pKa values, but MKP and DKP place the solution in different parts of that buffering curve:
- MKP buffers in the mildly acidic region and is often combined with other phosphates to fine-tune beverage or food pH.
- DKP buffers closer to neutral and weakly alkaline pH, which is particularly useful for protein-containing systems and certain industrial cleaners.
If a formulation requires pH around 3.5–4.5 (typical for many beverages), MKP fits naturally. If the target is around 7–9 (for protein stabilization or alkaline cleaning), DKP is usually the better tool.
2.2 Interaction with Proteins
The pH and ionic strength generated by phosphates directly influence protein solubility and stability.
- DKP (alkaline) is widely used in:
- coffee creamers and whiteners,
- RTD protein drinks,
- dairy and plant-based beverages,
- processed cheese and cheese analogues.
It helps keep proteins in a soluble, stable state, prevents coagulation and improves mouthfeel.
- MKP (acidic) can push proteins towards their isoelectric point in some systems, which may cause aggregation or precipitation if not carefully balanced with other ingredients.
2.3 Solubility and Clarity
Both MKP and DKP are highly water soluble, but their compatibility with other components differs:
- In acidified, clear beverages, MKP is often preferred because its acidic nature is compatible with low pH environments and can help maintain clarity.
- In near-neutral or slightly alkaline systems, DKP is easier to incorporate without compromising stability or clarity, assuming metals and impurities are well controlled.
2.4 Taste and Mouthfeel
Phosphates also have a sensory impact:
- DKP can contribute a slightly salty or alkaline note if dosed too high, but at controlled levels it improves creaminess and smoothness in coffee creamer and dairy-like beverages.
- MKP has a milder acidic character and is more “silent” from a taste point of view in acidified beverages where acids dominate the flavor profile.
3) Food & Beverage Applications: When to Use MKP vs DKP
In food and beverage applications, the choice between MKP and DKP is driven by pH targets, presence of proteins, clarity requirements and regulatory considerations.
3.1 Clear Beverages, Sports Drinks and Functional Drinks
For clear, acidified drinks:
- Preferred phosphate: MKP
Reasons:
- MKP supports the desired acidic pH range (typically 3.0–4.5).
- It provides potassium as an electrolyte source for sports and functional beverages.
- It helps stabilize pH without pushing the system towards turbidity or instability.
- Well-controlled food grade MKP can maintain clarity over shelf life if heavy metals and insolubles are kept low.
DKP is usually not suitable for such products, because its alkaline character can conflict with the acidified environment and may cause off-taste, precipitation or microbial stability challenges.
3.2 Dairy and Plant-Based Beverages, Coffee Creamers and RTD Protein Drinks
In systems containing proteins (milk proteins, whey, casein, soy protein, pea protein, etc.), avoiding precipitation and phase separation is crucial.
- Preferred phosphate: DKP
Typical roles of DKP in these applications:
- Shifting the pH away from the isoelectric point of proteins to maintain solubility.
- Acting as a buffer to resist pH drift during storage and heating.
- Complexing with calcium and other cations to improve protein stability and reduce sediment.
- Enhancing creaminess and mouthfeel in coffee creamers and whitener formulations.
Using MKP in these systems without proper balancing can move the pH closer to protein precipitation and lead to sediment, flocculation or unstable emulsions.
3.3 Processed Cheese and Cheese Analogues
In processed cheese, emulsifying salts are critical. While sodium-based phosphates are more common, potassium-based options like DKP may be used where sodium reduction is desired.
- DKP contributes to protein dispersion, meltability and texture.
- Its buffering capacity in the mildly alkaline range helps create the right environment for casein functionality.
MKP, due to its acidity, is not the primary phosphate for such applications.
3.4 Bakery and Leavening Systems
In chemical leavening, potassium phosphates may be part of baking powder systems, although sodium phosphates and other leavening acids are more common.
- MKP can contribute to the acidic side of the leavening reaction and provide K and P.
- DKP is rarely used as a leavening acid because of its alkaline character; it can reduce the effective acidity needed to react with bicarbonate.
Therefore, where potassium-based leavening is desired, MKP is often more relevant than DKP.
4) Agriculture & Fertigation: Why MKP Dominates
In crop nutrition, MKP and DKP are far from equal. One is a widely used specialty fertilizer; the other is rarely applied in large volumes.
4.1 MKP as a High-Value P–K Fertilizer
Monopotassium phosphate (MKP) is a standard water-soluble fertilizer, often labeled as 0–52–34 (P₂O₅–K₂O). It is used in:
- Fertigation and drip irrigation systems.
- Foliar spraying for fruit and vegetable crops.
- Greenhouse and hydroponic nutrient solutions.
Key advantages:
- High content of water-soluble phosphorus and potassium.
- Chloride-free, which is important for chloride-sensitive crops.
- Compatible with many other water-soluble fertilizers in tank mixes.
- Mildly acidic effect that can help improve nutrient availability in certain soils.
4.2 DKP in Agriculture
Dipotassium phosphate (DKP) is much less common as a fertilizer:
- Its nutrient ratio is less attractive compared to MKP for most P–K fertilizer strategies.
- Production costs and market structure make it less competitive for large-scale agricultural use.
- Its alkaline character is not always desired in fertigation systems, especially where slightly acidic solutions are preferred.
For most growers and fertilizer formulators, MKP is the clear choice, while DKP remains a niche or technically specific option rather than a mainstream fertilizer.
5) Industrial, Detergent and Process Applications
Outside food and agriculture, MKP and DKP are used in a range of industrial formulations, but again with different roles.
5.1 Detergents and Cleaning Products
In metal cleaning, CIP (clean-in-place), and some household or institutional cleaners:
- DKP is often used:
- as an alkaline buffer to maintain pH in the 8–10 range,
- to sequester hardness ions and improve cleaning efficiency,
- to help disperse soils and prevent redeposition.
MKP is less common in these alkaline detergents, because its acidic nature does not support the target pH profile.
5.2 Fermentation and Bioprocessing
In fermentation media and bioprocessing, phosphate salts supply both nutrients and buffering capacity.
- MKP is used where a slightly acidic pH or specific P–K balance is beneficial to the microorganism.
- DKP may be used where a more neutral to alkaline pH is required, or where the system benefits from additional potassium at higher pH.
The choice is driven by the biology of the organism, the target pH and the interaction with other media components.
6) Grade & Purity Considerations (Fertilizer, Feed, Food, Pharma)
For both MKP and DKP, applications are not defined only by chemistry. Grade and impurity control are equally important for safety and performance.
| Aspect | MKP (KH₂PO₄) | DKP (K₂HPO₄) |
|---|---|---|
| Fertilizer grade | Very common; major water-soluble P–K fertilizer | Rare; not widely used as a fertilizer |
| Feed grade | Used in some premixes and animal nutrition | Used in specific feed formulations as K and P source |
| Food grade | Used in beverages, some bakery and nutrition products | Widely used in coffee creamers, dairy, RTD protein drinks |
| Pharma / high purity | Special grades for pharmaceutical and biotech uses | Special grades for injectable and highly regulated uses |
| Key impurities to control | Heavy metals, fluoride, sulfate, insolubles, microbiology (for food/pharma) | Same as MKP; additionally critical for protein applications where trace metals may destabilize systems |
For sensitive applications (food, beverage, pharma, fermentation), buyers should always:
- Specify the grade (fertilizer / feed / food / pharma).
- Ask for detailed COAs covering metals, fluoride, sulfate, insolubles and microbiological parameters.
- Confirm that the phosphate is produced from suitable phosphoric acid routes and under appropriate GMP or food safety systems.
7) Practical Selection Guide: How to Decide Between MKP and DKP
For purchasing and formulation teams, the decision between MKP and DKP can be approached as a simple set of structured questions.
7.1 Key Questions to Ask Internally
- What is the target pH range of the final product?
- If it is clearly acidic (e.g. 3–4.5), MKP is usually the better fit.
- If it is near-neutral or slightly alkaline (7–9), DKP is often more appropriate.
- Does the formulation contain proteins?
- For dairy, plant-based beverages and protein drinks, DKP is usually preferred for stability.
- MKP is used more for nutrient supply and pH in non-protein beverages.
- Is the product clear or opaque?
- Clear beverages usually favor MKP for stability at low pH.
- Opaque, protein-rich systems (creamers, milky drinks) tend to favor DKP.
- What regulations and grade requirements apply?
- Fertilizer vs feed vs food vs pharma will strongly influence which suppliers and grades are acceptable.
7.2 Typical “Rules of Thumb” for Buyers
- Use MKP when:
- you need an acidic phosphate in beverages,
- you are formulating high-value P–K fertilizers,
- you want a potassium source for clear, low-pH drinks.
- Use DKP when:
- you need an alkaline buffer in dairy or plant-based beverages,
- you are formulating coffee creamer or protein drinks,
- you want to stabilize proteins and improve mouthfeel.
7.3 Common Mistakes to Avoid
Some of the most costly mistakes in phosphate purchasing happen when MKP and DKP are treated as interchangeable just because both contain potassium and phosphate.
- Replacing DKP with MKP in protein beverages, leading to coagulation or sediment.
- Using DKP in acidified clear drinks, resulting in off-taste or stability issues.
- Assuming fertilizer-grade MKP or DKP is acceptable for food uses without checking impurity limits.
- Not telling suppliers the exact application, so they quote a grade that is technically unsuitable even if it is cheaper.
Working with a specialized phosphate supplier that understands both chemistry and application requirements can dramatically reduce these risks.
8) FAQ: Key Questions from Buyers
Q1: Can I switch from MKP to DKP (or vice versa) just based on price?
No. MKP and DKP have different pH behavior and functional roles. Any substitution must be evaluated in the real formulation, and in many cases the two are not interchangeable at all.
Q2: Which one is better for sports drinks and electrolyte beverages?
In most cases, MKP is the better choice, because these products are acidified. MKP supports the required pH, provides potassium and is more compatible with clear beverage systems.
Q3: Which one is better for coffee creamers and dairy-like beverages?
DKP is generally preferred thanks to its alkaline buffering, protein-stabilizing effect and positive impact on mouthfeel. MKP typically does not provide the same functional benefits in these systems.
Q4: Do MKP and DKP share the same regulatory status in food?
Both can be used as food additives where permitted by local regulations, but the exact limits, purity requirements and labelling rules may differ by jurisdiction. Always consult relevant food codes (e.g. FCC, EU, GB) and work with suppliers who provide full documentation.
Q5: How can a specialist supplier help?
A knowledgeable professional supplier with multiple phosphate sources can:
- Match MKP or DKP grades to your application and regulatory needs.
- Explain why two offers with the same product name may have different quality and price.
- Provide suitable samples and multi-batch COAs for testing.
- Help you avoid hidden risks when switching suppliers or grades.
Instead of asking only for “MKP” or “DKP”, buyers get the best results when they clearly state how and where the phosphate will be used.